Novel cancer vaccines
What is a therapeutic cancer vaccine?
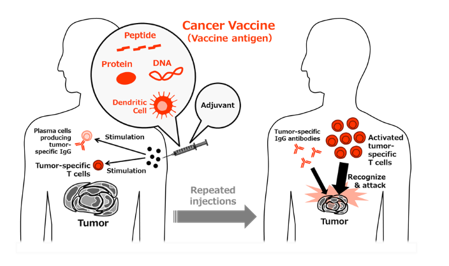
Therapeutic cancer vaccines contain antigens derived from tumor-specific antigen(s), and these vaccines are repeatedly administered to cancer patients to induce a tumor-specific immune response for tumor suppression. In most cases, cancer vaccines are composed of three components: a vaccine antigen, adjuvant, and delivery system. In recent years, numerous cancer vaccines containing various combinations of the components described below have been described and have been widely evaluated both in Japan and abroad.
Vaccine antigens: The antigens in cancer vaccines can include synthetic short or long peptides, recombinant proteins, or nucleic acids (mRNA/DNA) designed based on the amino acid sequences of tumor-specific antigenic proteins. In some cases, even tumor cells are used.
Adjuvants: In addition to traditional adjuvants, such as metal salts, saponins, and cytokines, agonists of Toll-like receptors (TLRs) and intracellular DNA/RNA sensors (STING, RIG-I, etc.) with potent innate immunostimulatory activity are currently attracting attention.
Delivery systems: Most commonly, these vaccine components are delivered using emulsions with depot effects (e.g., incomplete Freund’s adjuvant) in preclinical and clinical studies. However, liposomes, nanoparticles, and biological vectors, including bacteria and viruses, have recently emerged as efficient delivery system.
What we do: Generation of a novel, effective cancer vaccine system
We have been studying various cancer vaccine antigens, adjuvants, and delivery systems with an aim to establish a high-performance cancer vaccine technology with the ability to induce tumor-specific immune responses. 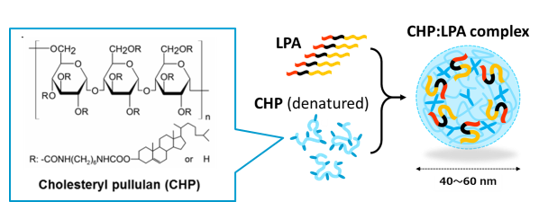 Through our research, we have found that the combination of a long peptide antigen, TLR agonist, and CHP nanogel delivery system is the most promising. Long peptide antigens are peptides with dozens of residues that can be produced by chemical synthesis. These peptides can contain a number of T cell epitopes that can induce various T cell responses. There is also no possibility of inducing immune tolerance, which is an important concern when using short peptide antigens. However, these long peptide antigens are prodrugs; therefore, it is necessary to excise the T cell epitopes contained in these long peptide antigens after uptake by antigen presenting cells, such as dendritic cells and macrophages. We have focused on the amino acid sequence between T cell epitopes in the long peptide antigen (the inter-epitope sequence), and we have identified multiple sequences that are efficiently cleaved when in antigen presenting cells. Using this long peptide design, stable induction of T cells becomes possible.
Through our research, we have found that the combination of a long peptide antigen, TLR agonist, and CHP nanogel delivery system is the most promising. Long peptide antigens are peptides with dozens of residues that can be produced by chemical synthesis. These peptides can contain a number of T cell epitopes that can induce various T cell responses. There is also no possibility of inducing immune tolerance, which is an important concern when using short peptide antigens. However, these long peptide antigens are prodrugs; therefore, it is necessary to excise the T cell epitopes contained in these long peptide antigens after uptake by antigen presenting cells, such as dendritic cells and macrophages. We have focused on the amino acid sequence between T cell epitopes in the long peptide antigen (the inter-epitope sequence), and we have identified multiple sequences that are efficiently cleaved when in antigen presenting cells. Using this long peptide design, stable induction of T cells becomes possible.
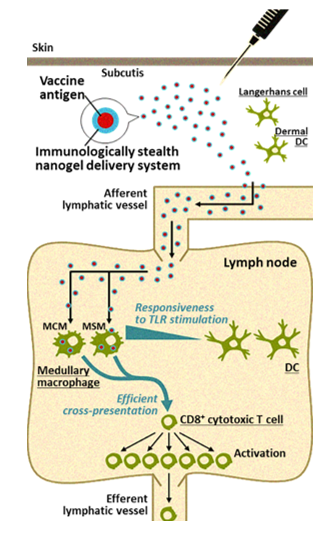
Cancer vaccines are often administered subcutaneously, intradermally, or intramuscularly. Regardless of the route of administration, subsequent efficient delivery of the vaccine antigens to the lymph nodes is very important for induction of specific immune responses. In collaboration with Kyoto University, we found that CHP is a unique polymeric material that is ideal for the purpose of efficient cancer vaccine delivery. CHP is a polysaccharide pullulan modified with cholesterol groups that spontaneously forms nano-sized stable gel particles, namely a nanogel. It is easy to embed vaccine antigens into CHP, and after subcutaneous injection, CHP nanogels containing long peptide antigens are rapidly and efficiently translocated into draining lymph nodes. This lymph node-oriented mode of transport is cell-independent. We also discovered that CHP nanogels are selectively taken up into medullary macrophages within the lymph nodes, a process that has not been well studied. Medullary macrophages exert potent cross-presentation activity in the presence of a TLR agonist. This medullary macrophage-oriented vaccine delivery system is very different from the others, and it greatly improves the immunogenicity of cancer vaccines. We have developed a new high-performance cancer vaccine consisting of a long peptide antigen, the CHP delivery system, and an adjuvant, and are keenly studying its clinical application.
What we do: Development of combination immunotherapy for the treatment of immune checkpoint inhibition-resistant tumors
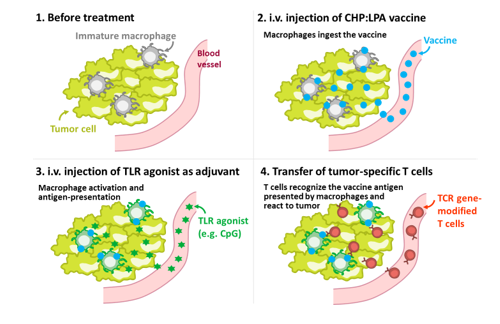
In recent years, various immune checkpoint inhibitors, such as anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies, have attracted great attention for their dramatic anti-tumor effects against various cancers. However, most tumors are refractory to this type of therapy. Therefore, more powerful, innovative immunotherapies are needed. We have established a mouse tumor model that is refractory to immune checkpoint inhibitors and we have analyzed these mice in detail. Our analysis showed that in refractory tumors, the local immune environment is inactive; specifically, the tumor-associated macrophages (TAMs) are immature and do not exhibit antigen presenting activity. Therefore, we attempted to deliver antigens to the TAMs using the macrophage-targeted vaccine delivery system contained in a CHP nanogel in the presence of a TLR agonist. Antigen delivery using this method activated TAMs and induced their antigen presenting activity. The treated tumors then became sensitive to the immune checkpoint inhibitors, and showed remarkable responsiveness to TCR-engineered T cell therapy. Therefore, we have designated a “TriCombo therapy,” consisting of three components: the TAM-targeted antigen delivery system using a CHP nanogel, a TLR agonist, and a genetically modified T cell therapy. We expect that this novel immunotherapy combination will lead to curable immune checkpoint inhibitor refractory human tumors. While further elucidating the underlying mechanism of action, we are also preparing for clinical evaluation of this TriCombo therapy.



 TOP
TOP